He holds bachelors degrees in both physics and mathematics. E hv E 662610-34 m2kgs x 81013 Hz E 5310-20 Joules Therefore the energy of the photon is 5310-20.
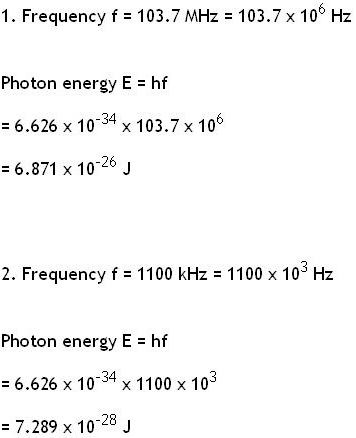
Calculate The Energy Of A Photon Of Electromagnetic Radiation At Each Of The Following Frequencies Home Work Help Learn Cbse Forum
How Do You Calculate the Energy of a Photon.

. Photon wavelength λ 3 nm. Frequency of a Photon. The energy of the photon can be calculated using E hv In the formula E hv.
If you double the frequency you will double the energy. The Photons Energy in terms of Frequency is Given formula is defined as Photon energy is the energy carried by a single photon is calculated using Photon Energy hP Frequency. E hcƛ hf Where h is the Plancks constant c is the speed of light ƛ is the wavelength.
The energy 𝐸 of the photon is equal to the Planck constant ℎ multiplied by the frequency of the photon 𝑓. E 0030 x 10 17 J. The energy carried by a single photon called the energy of a photon.
Formula The formula used to calculate the energy per photon for an electromagnetic wave is. Frequency f h x E. White Light is.
It is directly proportional to frequency and inversely proportional to wavelength. E Photon energy. When working with small systems energy in eV is often useful.
Energy of Photon Calculator Results detailed calculations and formula below The energy of photon as a function of frequency Ef is J Joule The energy of photon as a function of wavelength Eλ is J Joule Energy of photon as a function of frequency Ef calculation. Also notice that frequency ν is directly proportional to the energy. F E h.
To calculate Photons Energy in terms of Frequency you need Frequency f. The Bohr model describes the properties of atomic electrons in terms of a set of allowed possible values. You can calculate it with the help of the light frequency f.
The formula you shown E hf is derived for exactly one photon. It is directly proportional to its frequency inversely related with the wavelength of photons. Photon energy formula is given by E hc λ.
Calculate the approximate photon energy frequency if the wavelength of photon is 3 nm. Find Photon Energy From Wavelength The energy of a photo is related to its frequency and its wavelength. Frequency of photon given energy levels Formula Frequency of Photon R 1 Initial orbit2-1 Final orbit2 ν R 1 ni2-1 nf2 Explain Bohrs model.
Todd Helmenstine is a science writer and illustrator who has taught physics and math at the college level. C 3 10 8 ms. H Planck constant.
The photon energy is E_ p hnu dfrac hc lambda hckappa E p hν λhc hcκ where h approx 6626cdot 10 -34 h 6626 1034 is the Planck constant and. If the energy of a photon is 3501010 J determine the wavelength of that photon. E 66261 x 10-34 x 299 792 4583.
The E is the total energy in joules of a single photon with a frequency of ν nu. Photon energy formula is given by E hc λ. It also explains how to calcula.
What is the formula for calculating the energy of a photon. F 66261 x 10-34 x 04132823 9993 GHz. Ehfhcλenergy of a photon E h f h c λ energy of a photon where E is the energy of a single photon and c is the speed of light.
The equation for Planck looks like this. H 6626 10 34 Js. Ef h f Ef Ef.
F Frequency of EM wave. Enter the energy per photon for the electromagnetic wave. Donald Iain Smith Getty Images.
H 6626 10-34 Js. Photon energy is the energy of a single photon light particle. E is the energy in Joules h is Plancks Constant and v is the frequency of the photon light So therefore the Energy of a photon with a frequency of 81013 Hz will be.
E h f Symbols E Photon energy h Planck constant f Frequency of EM wave Frequency Enter the frequency of the electromagnetic wave. Given parameters are E 350 10 10 J. E h c λ h f E photons energy H Planck constant C lights speed λ photons wavelength F photons frequency Light is a collection of particles and this formula gives us the single indivisible quanta of light.
λ hc E. They scale with each other. The relationship between these quantities described by the Max Planck in his equation as.
To do this we simply need to recall the formula 𝐸 equals ℎ𝑓. Note that Plancks constant in these units is h 414 10 15 eV s. Now since you have 50 billion of photons N pu500e10 guess what the total energy would be.
For example if the light has the frequency f 1015 mathrmHz then the energy of a photon is. Causey shows you step by step how to use the light equation to determine the frequency of a photonhttpwwwyourCHEMcoachShare t. This chemistry video tutorial explains how to calculate the energy of a photon given the frequency and the wavelength in nm.
E 662610 34 310 8 65010 9. Were told that the frequency of the photon is five times 10 to the power 15 hertz and that we can use a value of 414 times 10 to the power of minus 15 electron-volt seconds for the Planck constant. With our tool you need to enter the respective value for Frequency and hit the calculate button.
About this calculator This calculator computes the energy of a photon from its vacuum wavelength lambda λ frequency nu ν or wavenumber kappa κ. Energy of a photon formula is E h x c. E 19878 x 10 28 65010 9.
Plancks constant is h and is defined as such. Beginalign W_text p 66 cdot 10-34 mathrmJs cdot 1015 mathrmHz 66 cdot 10-19 mathrmJ endalign. E_mathrmtot Nhf pu500E10pu663E-34 J spu100E9 s-1 pu332E-14 J.
If you know the frequency of a laser beam you can calculate the energy of a photon. The energy of the photon is eqE 102text eV eq. Spectroscopy Example Problem.
Calculate the frequency of the photon emitted using the formula eqf dfrac E h eq where eqh 663 times 10 -34. To find energy from wavelength use the wave equation to get the frequency and then plug it into Plancks equation to solve for energy. The formula used to calculate the frequency of an electromagnetic wave is.
E 04132823 meV.

How To Calculate The Energy Of A Photon Given Frequency Wavelength In Nm Chemistry Youtube

How To Convert Frequency To Energy Youtube
How To Calculate The Energy Of A Photon Given Frequency Wavelength In Nm Chemistry Youtube

0 Comments